Fine-controlled in vitro percutaneous system KX-V/HDP
1, Temperaturecontrollable (30~50±0.2°C);
2. Controllablespeed (300~1200±5% RPM);
3. Adjustable testmode (KX-10VPC / horizontal KX-5HPC conversion);
4. In line withthe FDA published local preparation BE replacement technical requirements for in vitro test and methodologicalresearch;
5. According totemperature and receptor solution’s viscosity, determine the speed, the datacan be obtained accurately reflect the skin's transdermal absorption of thedrug;

The precision controledlaboratory coating machine KX-FC-19HP
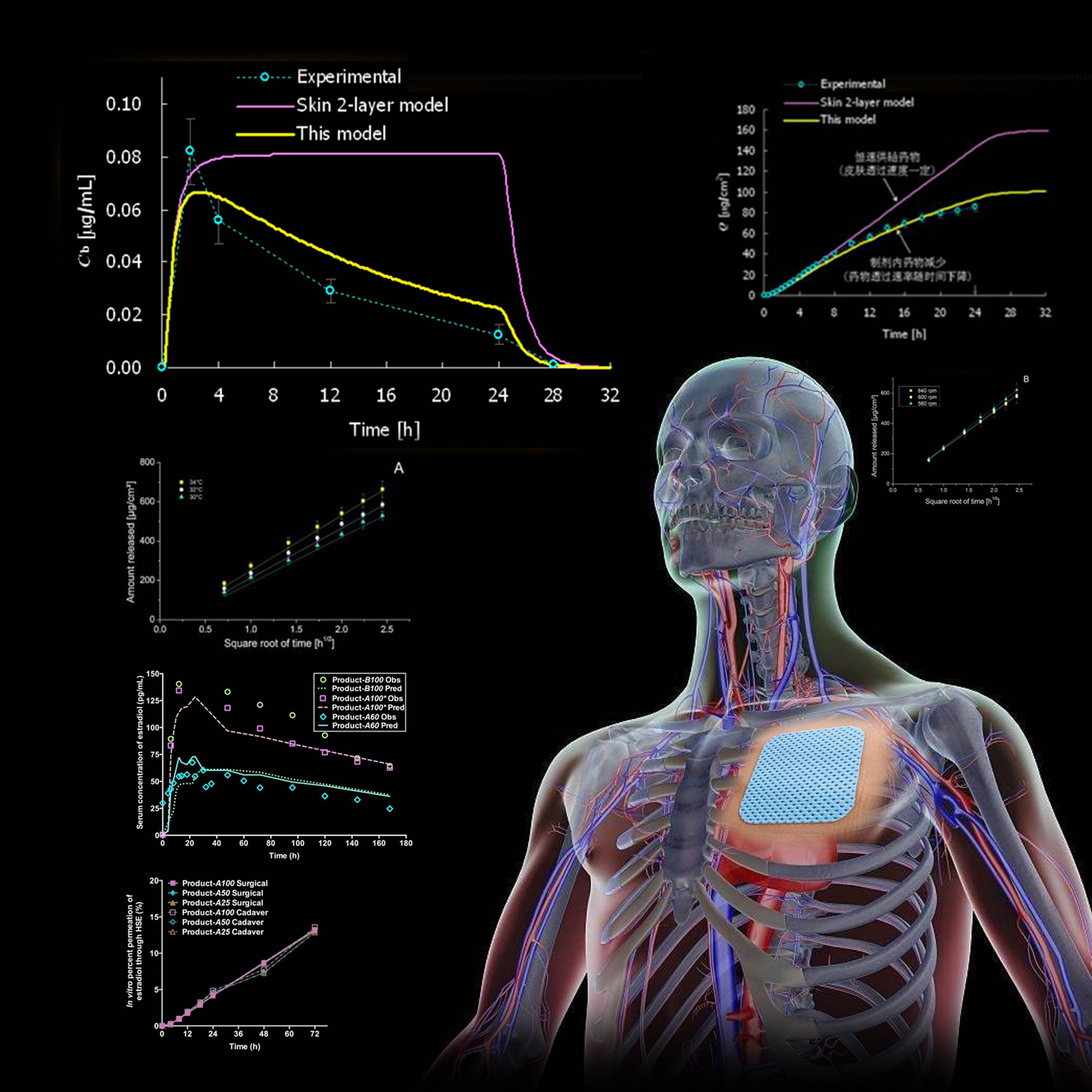
In vitro percutaneous permeation data analysis and TTS optimization systemTRANSDERMAL-CAD
|
|